- Cement is a vital ingredient in concrete—the world’s most widely used building material. It binds together sand, gravel, and crushed stone, giving concrete its strength and durability.
- But this widespread use comes at a cost: cement production contributes about 8% of global carbon dioxide emissions, due to both chemical reactions and the burning of fossil fuels.
- Roughly 60% of these emissions come from process emissions—the CO2 released during the chemical reaction that converts limestone (calcium carbonate) into lime (calcium oxide).
- The remaining 40% comes from combustion emissions, as fossil fuels—often coal—are burned to heat cement kilns to temperatures exceeding 1400°C.
- Strategies to reduce CO2 emissions associated with concrete include improving the efficiency of cement production, using alternative fuels in kilns, partially replacing cement with other less-polluting binding materials, and recycling concrete to reduce demand for new cement.
- One emerging approach to reduce emissions is carbon capture and storage (CCS), which involves separating CO2 from cement plant exhaust gases before they are released, and storing it permanently underground.
- Implementing CCS in cement manufacture will be expensive, and the infrastructure needed to transport and store captured CO2 is not yet available in most parts of the world.
- However, the imminent commissioning of CCS facilities at the Brevik Cement Plant in Norway—and the transport of captured CO2 to the completed storage site on Norway’s west coast—will serve as a demonstration of how carbon capture can be integrated into cement production at scale.
Cement manufacturing emits CO2 from two main sources. About 60% of the emissions arise from calcination, the chemical process in which limestone (CaCO₃) is heated to produce lime (CaO), releasing CO2.
- Combustion emissions: About 40% of emissions come from burning fossil fuels, typically coal, to reach the extremely high temperatures needed for cement production. These high temperatures, often exceeding 1400°C (2550°F), are essential for breaking down raw materials and triggering the chemical reactions that form cement. The reliance on fossil fuels for this energy-intensive process results in substantial CO2 emissions.
- Process emissions: The remaining 60% of emissions result from calcination, a chemical reaction that converts limestone (CaCO3) into lime (CaO), a key ingredient in cement. This process inherently releases CO2 as a byproduct. The chemical equation for this reaction is CaCO3 → CaO + CO2. This means that even if the energy used in cement production were completely decarbonized, there would still be significant CO2 emissions from the process itself. These two sources together make cement production highly carbon-intensive. As demand for construction materials increases globally, especially in developing countries, addressing these emissions has become a critical challenge in the fight against climate change.
The simplified chemical reaction is:
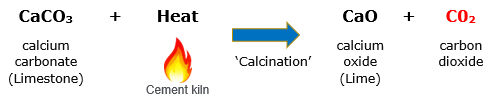
Calcination in cement manufacturing involves heating limestone to produce lime (CaO), with CO2 as a by-product.
This reaction produces about 60% of CO2 emissions, while the remaining 40% arises from burning coal to achieve the high temperatures required.
This reaction is a fundamental part of cement production and generates process emissions—emissions that occur regardless of the energy source.
The remaining emissions come from the combustion of fuels—typically coal, petcoke, or natural gas—used to heat kilns to around 1450°C. These are known as combustion emissions. While these can be reduced by using alternative fuels or renewable energy, process emissions remain a persistent challenge.
Reducing the environmental footprint of concrete involves strategies targeting both its key ingredient—cement—and the concrete mix as a whole.
Strategies for greener concrete include:
- Recycled aggregates: Using crushed concrete from demolition reduces demand for virgin aggregate and lowers emissions from extraction and transport.
- Efficient design and use: Optimising structural design can reduce the volume of concrete needed, and thus the amount of cement required.
- Carbon-absorbing concretes: New formulations are being developed that absorb CO2 from the air after they are set in place, offering potential for net-negative emissions over the material's lifespan.
Strategies for greener cement focus on reducing the emissions associated with producing clinker and using alternative materials:
- Clinker substitution: Incorporating supplementary cementitious materials (SCMs) to replace part of the clinker in cement.
- Why SCMs are suitable: SCMs can act as binding agents similar to clinker but without the need for CO2-emitting calcination.
- Why SCMs are greener: They often come from industrial by-products or naturally occurring materials that require less energy to process and generate far fewer emissions.
- Low-carbon cement formulations: New cement types that require less or no limestone are under development, aiming to reduce or eliminate calcination-related emissions.
- Carbon capture: Capturing and storing or reusing CO2 emitted during cement production, particularly from calcination, is essential to dealing with residual emissions that other strategies cannot address.
Because calcination emissions are intrinsic to clinker production, carbon capture is one of the few viable options for addressing them. By capturing CO2 from the flue gas before it enters the atmosphere, and then either storing it (CCS) or reusing it (CCU), producers can mitigate the climate impact.
Post-combustion capture, the most widely used method, involves absorbing CO2 from kiln exhaust gases using a chemical solvent. The gas is later separated, compressed, and either stored or repurposed. This method can be retrofitted to existing plants.
Alternative approaches include oxy-fuel combustion, where pure oxygen replaces air in the combustion process. This produces a flue gas that is mostly CO2 and water vapour, simplifying CO2 separation.
Pilot and commercial-scale projects are testing carbon capture in cement production.
Several projects are underway, demonstrating the growing interest in and feasibility of implementing carbon capture technologies in the cement industry:
- The Norcem Brevik Project, Norway: Operated by Heidelberg Materials, this project aims to capture up to 400,000 tonnes of CO2 annually from their cement plant in Brevik, representing approximately 50% of the plant's emissions. The project uses amine scrubbing technology and is part of a larger CCS initiative in Norway. The captured CO2 will be liquefied and shipped to an offshore storage site on Norway's west coast. This project is significant as it's set to be the world's first industrial-scale CCS project in cement production.
- The Padeswood CCS Project, UK: Led by Hanson Cement (part of the HeidelbergCement Group), this project is part of the larger HyNet North West cluster, a comprehensive low-carbon industrial zone. The project plans to capture CO2 emissions from the Padeswood cement plant and transport them via pipeline to depleted gas fields under the North Sea for permanent storage. The project aims to capture around 800,000 tonnes of CO2 annually, representing about 85% of the plant's emissions.
- The LEILAC-2 Demonstration Project, Germany: This project, led by technology company Calix, is developing a direct separation process that captures CO2 released during limestone calcination. The LEILAC (Low Emissions Intensity Lime And Cement) technology uses a specially designed reactor that keeps the CO2 released during calcination separate from other gases, potentially increasing capture efficiency and reducing energy requirements. The LEILAC-2 project aims to test this technology at commercial scale, targeting a 20% reduction in emissions.
- The Carbon Dioxide Full Oxygen Combustion Enrichment and Purification Demonstration Project in Qingzhou, Shandong Province, China, is the world’s largest oxy-fuel carbon capture initiative in the cement sector. Developed by Qingzhou Zhonglian Cement Co., Ltd., the facility began operations in January 2024 and is designed to capture up to 200,000 tonnes of CO2 per year.
The pure oxygen oxy-fuel combustion process produces a CO2-rich exhaust stream, lowering capture costs. The captured CO2 is used for enhanced oil recovery (EOR) and agricultural applications.
Carbon capture and storage (CCS) could play a big role in cutting emissions from cement production—but putting it into practice overcoming many hurdles, from high costs to missing infrastructure. Some of the main challenges include:
- High costs: Installing and operating capture systems requires significant capital and energy.
- Energy requirements: Capture processes consume extra energy, affecting plant efficiency.
- Space constraints: Older plants may lack the space for bulky capture units.
- Gas purity: Dust and contaminants in cement kiln gases can interfere with capture systems.
- Infrastructure gaps: CO2 transport and storage networks are underdeveloped in many regions.
- Regulatory uncertainty: A lack of stable carbon pricing and policy incentives can deter investment.
See also: Projects | Research | Latest Updates | Glossary